POLYMERIC FUEL CELLS
Proton Exchange Membrane Fuel Cell – PEMFC
Polymer Electrolyte Fuel Cell – PEFC
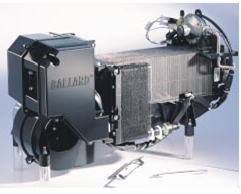
Commercial module NEXATM by Ballard (1.2 kW)

PEMFC del modelo NECAR 5 de Daimler-Chrysler
A bit of History
In 1962 General Electric (GE), under the direction of Thomas Grubb and Leonard Niedrach developed the first polymer membrane electrolyte, which is the first milestone in this type of fuel cells. The GE stacks replace the batteries used in the previous Mercury Project in the Gemini Project. A new polymer formulation developed by Grot in 1972 improved the characteristics and durability of the electrolyte and, later, in 1986, Raistrick made major improvements in manufacturing processes. In 1981 Ballard was founded, which began the development of its models in 1983 and in 1989 produced the first conceptual demonstration units. Between 1992 and 1994, they built functional prototypes and in 2001 launched its first NexaTM commercial module.

Thomas Grubb (left) and Leonard Niedrach (right) next to a polymer membrane fuel cell.

Roy Mushrush, at the time, director of the Department of Energy Conversion of GE, showing part of the 96 single cells of the stack of the image. The Gemini Protecto ships incorporated three of these modules.

