INTRODUCTION
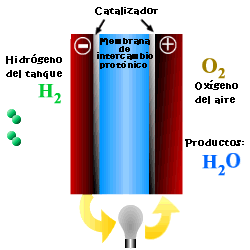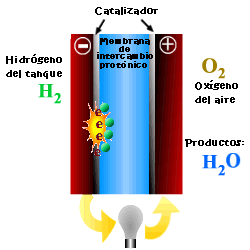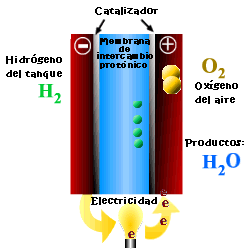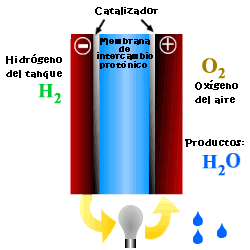
A cell or fuel cell is an electrochemical device that converts the chemical reaction energy directly into electrical energy, while supplying fuel and oxidant to its electrodes, with no other limitations than the processes of degradation or malfunction of the components. As a result of the electrochemical reaction, water and electricity are produced. The water leaves the fuel cell through the electrodes and the electric current passes to an external circuit.
In principle, any substance susceptible to chemical oxidation, which can be supplied continuously to the cell, can be used as fuel. Likewise, any substance that chemically reduces quickly enough can serve as an oxidant. Hydrogen and gaseous oxygen are the fuel and oxidant chosen in most fuel cell applications. Hydrogen presents a high reactivity in the presence of suitable catalysts, can be obtained from hydrocarbons, and reaches a high energy density when stored cryogenically for applications in closed environments. Oxygen is obtained directly from the air and its storage is easy and economical.
Fuel cells differ from batteries in that the latter are energy storage devices. The maximum available energy is determined by the amount of chemical reagents stored inside the battery itself that will stop producing energy when the chemical reagents are consumed. In a secondary battery, chemical reagents are regenerated by recharge, which involves putting energy from an external source into the battery.
FUNCTIONING
The global reaction that takes place in a fuel cell is the combination of hydrogen and oxygen to form water:
2H2+O2 —> 2H2O
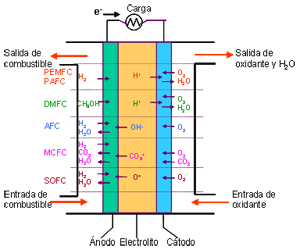
Unlike conventional combustion, in fuel cells, comburent and fuel do not come into direct contact. The oxidation of the fuel takes place at the anode (oxidation half-reaction), while at the cathode the oxidant consumes the positive ions of the electrolyte and the electrons generated at the anode (reduction half-reaction), which are moved by the difference in potential generated between the two electrodes.
The basic elements of a fuel cell are the electrodes, the cathode, positive pole, and the anode, negative pole; the electrolyte, the substance responsible for transporting the ions produced in the redox reactions; the matrix, which contains the electrolyte and which is not necessary when it is solid; and the bipolar plate, which acts as a current collector and gas distributor.
In the operation of the cell, the gases pass through the electrolyte through the pores of the electrodes, the oxidants through the cathode and the fuels through the anode. An electrode-electrolyte-reactive interface is produced that plays a determining role in the electrochemical behavior of the cell. At this interface, a very thin region a few microns thick, the gas diffuses to the surface of the electrode wetted by the electrolyte, where it reacts electrochemically producing the electrical charges that create the external electrical current, and the ions that are transported through of the electrolyte closing the circuit. The amount of electrolyte present in the interface is a key factor. An excessive electrolyte content in the interface makes it difficult for the gases to reach the reactive centers, where the catalyst is located, while a too low electrolyte content at the interface limits the transport of the ions to the places where the reaction takes place. .
HISTORY (The origins of Fuel Cells)

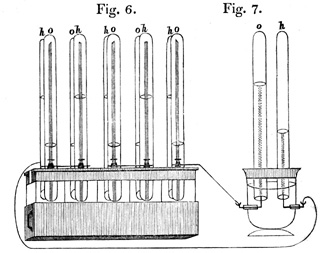
The original idea dates back to 1839, when a Welsh jurist, Sir William Robert Graves (1811-1896), designed the first device. For its preparation, it used two platinum electrodes submerged in sulfuric acid, which fed with oxygen and hydrogen, respectively.
From the dissociation of H2SO4, the reduction took place in the electrode fed with O2 (cathode), which reacted with the H+ ions forming water; in this reaction the electrons intervened, which were generated at the anode during the oxidation of H2, which reacted with the SO42- ion to form sulfuric acid.
Connecting six of these devices in series, he used them as an electrical generator to decompose the water. Subsequently, other inventors were introducing certain modifications, thus, in 1855, Becquerel built another cell that consumed carbon from a molten nitrate electrolyte (potassium nitrate) contained in a platinum container.
In 1894, Mond and Langer used platinum plates with small holes as electrodes, which they coated with platinum black, using as electrolyte diluted sulfuric acid within a porous matrix called paris.
Configuration of the William R. Grove device, according to its publication
«On the Gas Voltaic Battery», Philosophical Magazine and Journal of Science (1843) p. 272
But the true technological development of these devices took place, thanks to Francis T. Bacon, already entered the twentieth century. In 1952 a 5 kW plant was built based on the hydrogen / oxygen technology that had been developed since 1932. The cell consisted of a nickel anode, a lithium nickel oxide cathode, and a potassium hydroxide electrolyte. concentrated at 85%; operated at a temperature of 200-240 ° C, and at a pressure of 30-40 bar that prevented boiling of the electrolyte. This pile served as the basis for developing the auxiliary energy sources of space vehicles. But it has been due to the global energy crisis that was experienced from 1973, when there has been an increase in the study for the development of fuel cells.
For more information on the history of fuel cells, see the Fuel Cell Project page of the Smithsonian Institute
(http://americanhistory.si.edu/fuelcells).
Types of Fuel Cells
— POLYMERIC FUEL CELLS
Proton Exchange Membrane Fuel Cell – PEMFC
Polymer Electrolyte Fuel Cell – PEFC
— PHOSPHORIC ACID FUEL CELL or PAFC

